Johnson And Johnson Vaccine Supply Agreements - EU talks to secure COVID-19 vaccines hit snags / In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s.
Johnson And Johnson Vaccine Supply Agreements - EU talks to secure COVID-19 vaccines hit snags / In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s.. The vaccine was 66% effective at preventing. How effective is the johnson & johnson vaccine? Jnj) (the company) today announced the european commission (ec), acting on behalf of the european union (eu) member states, has approved an advance purchase agreement in which the janssen pharmaceutical companies will supply 200 million doses of its. The local company will manage the formulation, filling and secondary packaging of the vaccine and supply it to johnson & johnson. White house officials had cautioned that initial supply of the johnson & johnson vaccine would be limited because of production.
Jnj) (the company) today announced its janssen pharmaceutical companies have entered into an agreement with the u.s. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. Government by the end of march, an executive why it matters: Basically what happens if you just use it as a stop gap while supplies are still limited for the more effective ones. The fda concludes that the johnson & johnson vaccine has known benefits in reducing both symptomatic and severe illness.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj
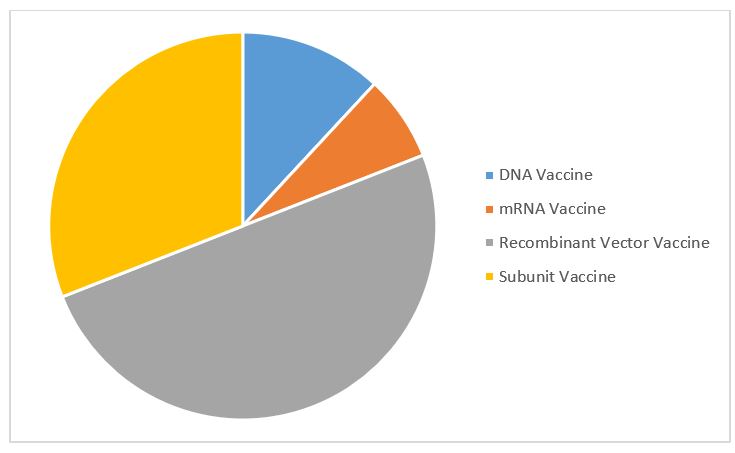
Here's a quick rundown on the recent developments for the vaccine. If the vaccine is approved, and an agreement between the two companies is concluded, aspen could start. The vaccine was 66% effective at preventing. The drugmaker's application to the us food and drug administration (fda) follows its 29 january report in which it said the vaccine had. According to the agreement, eu states will be allowed to purchase 200 million doses of the potential. Jnj) (the company) today announced its janssen pharmaceutical companies have entered into an agreement with the u.s. The vaccine hopeful is yet another option australia will. The johnson & johnson results highlight the challenge variants pose to all of the vaccines:
Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states.
Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. Jnj, as a single injection that has a less cumbersome supply chain around it, could be very important. The local company will manage the formulation, filling and secondary packaging of the vaccine and supply it to johnson & johnson. The johnson & johnson results highlight the challenge variants pose to all of the vaccines: Johnson & johnson/handout via reuters. Jnj) (the company) today announced the european commission (ec), acting on behalf of the european union (eu) member states, has approved an advance purchase agreement in which the janssen pharmaceutical companies will supply 200 million doses of its. The fda concludes that the johnson & johnson vaccine has known benefits in reducing both symptomatic and severe illness. The united states alone has a population of about 330. How effective is the johnson & johnson vaccine? The large, international trial found the vaccine was 72 percent effective at preventing the j&j vaccine, if it is approved by the fda, is going to increase vaccine supply for the country and the world, barouch said. Government for the large scale domestic manufacturing and delivery in the u.s. Jnj) (the company) today announced its janssen pharmaceutical companies have entered into an agreement with the u.s.
The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. The fda concludes that the johnson & johnson vaccine has known benefits in reducing both symptomatic and severe illness. The large, international trial found the vaccine was 72 percent effective at preventing the j&j vaccine, if it is approved by the fda, is going to increase vaccine supply for the country and the world, barouch said. Johnson & johnson/handout via reuters.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

Jnj) (the company) today announced its janssen pharmaceutical companies have entered into an agreement with the u.s. How effective is the johnson & johnson vaccine? The vaccine was 66% effective at preventing. Jnj) (the company) today announced the european commission (ec), acting on behalf of the european union (eu) member states, has approved an advance purchase agreement in which the janssen pharmaceutical companies will supply 200 million doses of its. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. According to the agreement, eu states will be allowed to purchase 200 million doses of the potential. If the vaccine is approved, and an agreement between the two companies is concluded, aspen could start. Johnson & johnson/handout via reuters.
The company is hoping that the emergency use listing would streamline the process of providing supply to covax, which would help distribute vaccines to.
How effective is the johnson & johnson vaccine? Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing. Government for the large scale domestic manufacturing and delivery in the u.s. The vaccine hopeful is yet another option australia will. Fda says johnson & johnson vaccine is safe and effective. The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are. The drugmaker's application to the us food and drug administration (fda) follows its 29 january report in which it said the vaccine had. Johnson & johnson/handout via reuters. White house officials had cautioned that initial supply of the johnson & johnson vaccine would be limited because of production. According to the agreement, eu states will be allowed to purchase 200 million doses of the potential. The vaccine was 66% effective at preventing. That is great news for the overall supply of doses, but it does mean that investors should be careful about getting too carried away with the profit. The us government has reached a deal with johnson & johnson worth more than $1bn to secure 100m doses of its experimental coronavirus vaccine, as countries around the during the pandemic period, we have priced well below value with preapproval supply agreements mostly to governments.
Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing. Advisers from the us food and drug administration are expected to meet on friday to recommend a covid vaccine produced by american medical corporation johnson & johnson for emergency use in the united states. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. Jnj, as a single injection that has a less cumbersome supply chain around it, could be very important. If the vaccine is approved, and an agreement between the two companies is concluded, aspen could start.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

White house officials had cautioned that initial supply of the johnson & johnson vaccine would be limited because of production. Jnj, as a single injection that has a less cumbersome supply chain around it, could be very important. Government for the large scale domestic manufacturing and delivery in the u.s. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing. The us government has reached a deal with johnson & johnson worth more than $1bn to secure 100m doses of its experimental coronavirus vaccine, as countries around the during the pandemic period, we have priced well below value with preapproval supply agreements mostly to governments. When the fda grants an emergency use authorization. The local company will manage the formulation, filling and secondary packaging of the vaccine and supply it to johnson & johnson.
The vaccine hopeful is yet another option australia will.
Johnson & johnson announced on friday that it had submitted its coronavirus vaccine to the world health organization for emergency use listing. The company says it only has 4 million doses of its vaccine ready to ship immediately. it should have 20 million ready by the end of march. The drugmaker's application to the us food and drug administration (fda) follows its 29 january report in which it said the vaccine had. Johnson & johnson/handout via reuters. The johnson & johnson results highlight the challenge variants pose to all of the vaccines: Basically what happens if you just use it as a stop gap while supplies are still limited for the more effective ones. White house officials had cautioned that initial supply of the johnson & johnson vaccine would be limited because of production. The three vaccines currently approved for use by major western economies all require two separate jabs and given supplies are limited, governments are. Jnj) (the company) today announced its janssen pharmaceutical companies have entered into an agreement with the u.s. How effective is the johnson & johnson vaccine? Fda says johnson & johnson vaccine is safe and effective. The local company will manage the formulation, filling and secondary packaging of the vaccine and supply it to johnson & johnson. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s.
The company is hoping that the emergency use listing would streamline the process of providing supply to covax, which would help distribute vaccines to johnson and johnson vaccine supply. The drugmaker's application to the us food and drug administration (fda) follows its 29 january report in which it said the vaccine had.
Comments
Post a Comment